Clinical Imaging
Clinical Imaging and Harmonic Generation Microscopy
UltraFast Optics (UFO) laboratory, led by Prof. Chi-Kuang Sun, has been working on development of the Harmonic Generation Microscopy System (HGM) since the early 2000s, including the Second and Third Harmonic Generation, and more recently the Spectral Third Harmonic Generation (sTHG) Microscopy and the Third Harmonic Generation Enhancement Ratio (erTHG) Microscopy systems. The goal is to device a novel cutting-edge optic system capable of conducting live in vivo imaging deep inside the human body with a super-resolution without fluorophores. In 2003, UFO demonstrated that the HGM system has the capacity to noninvasively view the embryonic morphological changes and complex developmental processes at the cellular and subcellular levels in live zebrafish with a penetration more than 1.5 mm, a resolution less than 500 nm, and for continuously 3 days without any damage or delay on the development. Furthermore, our team was the first to propose using the HGM system to conduct virtual skin biopsy in human, and subsequently demonstrated in multiple clinical trials that the HHGM system is proficient to perform in vivo virtual skin biopsy. This allowed for identification of different skin diseases based on traditional histopathological characters as well as some new information such as melanin distribution, melanocyte dendricity, and the collagen fiber texture. On top of that, the HGM system could also provide data for differential diagnosis involving nonmelanoma pigmented skin lesions. Examination of the molecular structure in people of different age groups also revealed age-associated molecular changes in the skin tissues; including cellular and nuclear size of the basal keratinocytes, depth of the dermal papilla zone, height of isolated dermal papilla and the three-dimensional interdigitation index.
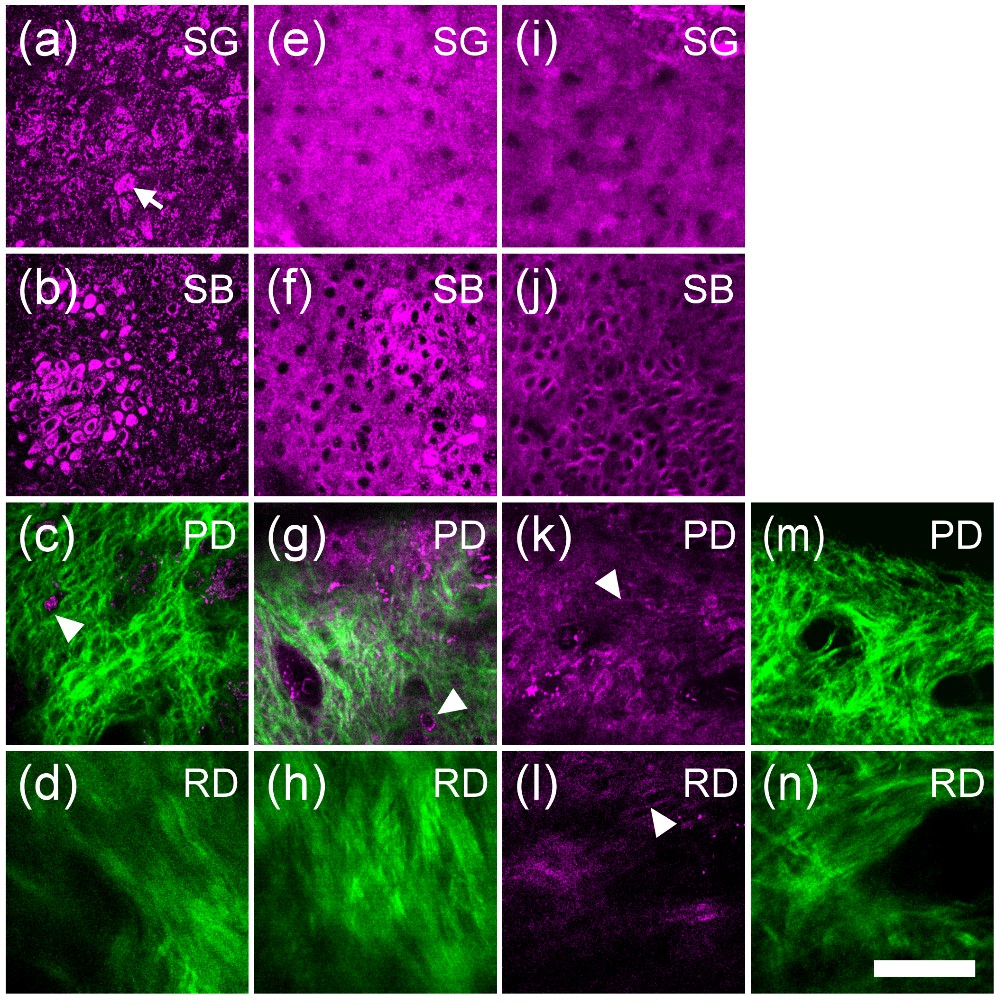
In vivo HGM images of the (a)–(d) African-American skin (photo type VI), (e)–(h) Asian skin (photo type III), and (i)–(n) Caucasian skin (photo type I) obtained at SG, SB, PD, and RD. In the SG layer, the scattered bright spots (arrows) with stronger THG contrast can be found in (a) dark skin, while the cytoplasm of the SG cells in (e) Asian skin and (i) light skin are shown with a uniform THG brightness. At dermo-epidermal junction, THG-brighter basal cells (relative to surrounding SS cells, arrowheads) were found in (b) dark skin and (f) Asian skin but not in (j) light skin. In PD, the inactivated melanocytes (arrowheads) can often be found in (c) dark skin and (g) Asian skin but not in light skin (epi-THG (k); epi-SHG (m)]. Moreover, in RD [arrowhead in (l)] elastic fibers can be revealed through epi-THG microscopy. Epi-SHG and epi-THG are represented by green and purple pseudo-colors. Scale bar: 50 mm. SG: Stratum Granulosum, SB: stratum basale, PD: papillary dermis, RD: reticular dermis. (Chen et al., 2010)

Harmoscope: A clinical HHGM system prototype
These findings collectively demonstrate the value of the HGM system as a tool with broad spectrum biological applications. It can be used as a medical device for skin-related disease monitoring, including diagnostics, disease and treatment progression without the need of invasive surgical procedures on the patients. Moreover, this diagnostic procedure eliminates the tedious fixing and staining steps necessary in the conventional skin biopsy approach, as well as the false results obtained as a consequence of inappropriate handling of samples. It can also be used to examine developmental and structural biology in vivo, thereby providing insights into previously unseen biological phenomena. All these properties are made possible by its increased penetrability and spatial resolution (300-400 nm), and most importantly, its use was shown to be safe without compromising the viability of both zebrafish and mouse embryos.
Current work in progress includes noninvasive treatment assessment with histopathological details, immediate border definition during surgery, expanding the investigation of physiological changes associated with disease progression, such as angiogenic events and tumour induced hypoxia using the HGM, and to further advance the function of the photonic device, including advanced light source, to incorporate 3D visual systems, and to be combined with a deep learning AI system. The aim is to evaluate known disease related alterations using the HGM system, which can be incorporated into future diagnostic routines using this system.
References
C.-K. Sun, C.-C. Chen, S.-W. Chu, T.-H. Tsai, Y.-C. Chen, and B.-L. Lin, “Multiharmonic generation biopsy of skin,” Optics Letters 28 (24), pp. 2488-2490 (2003).
C.-K. Sun, S.-W. Chu, S.-Y. Chen, T.-H. Tsai, T.-M. Liu, C.-Y. Lin, and H.-J. Tsai, “Higher harmonic generation microscopy for developmental biology,” Journal of Structural Biology 147 (1), pp. 19-30 (2004).
S.-Y. Chen, S.-U. Chen, H.-Y. Wu, W.-J. Lee, Y.-H. Liao, and C.-K. Sun, “In Vivo Virtual Biopsy of Human Skin by Using Noninvasive Higher Harmonic Generation Microscopy,” IEEE Journal of Selected Topics in Quantum Electronics 16 (3), pp. 478-492 (2010), Invited Paper.
Y.-H. Liao, S.-Y. Chen, S.-Y. Chou, P.-H. Wang, M.-R. Tsai, and C.-K. Sun, “Determination of chronological aging parameters in epidermal keratinocytes by in vivo harmonic generation microscopy,” Biomedical Optics Express 4 (1), pp. 77-88 (2013).
Y.-H. Liao, W.-C. Kuo, S.-Y. Chou, C.-S. Tsai, G.-L. Lin, M.-R. Tsai, Y.-T. Shih, G.-G. Lee, and C.-K. Sun, “Quantitative analysis of intrinsic skin aging in dermal papillae by in vivo harmonic generation microscopy,” Biomedical Optics Express 5 (9), pp. 3266-3279 (2014).
M.-R. Tsai, Y.-H. Cheng, J.-S. Chen, Y.-S. Sheen, Y.-H. Liao, and C.-K. Sun, “Differential diagnosis of nonmelanoma pigmented skin lesions based on harmonic generation microscopy,” Journal of Biomedical Optics 19 (3), 036001 (2014).

